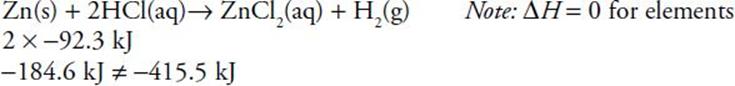
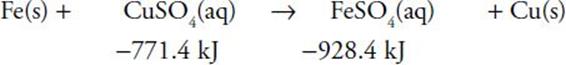
in the first reaction , 2 moles of HCl have ΔH = 2 × −92.3 kJ. zinc chloride has ΔH = −415.5 kJ. This comparison leaves an excess of 230.9 kJ of heat given off, so the reaction would occur. in the second reaction−928.4 − (−771.4) = −157.0 kJ, which is the excess to be given off as the reaction occurs:
我看不懂這在幹嘛 為甚麼要相減 要怎麼判段reaction會發生
