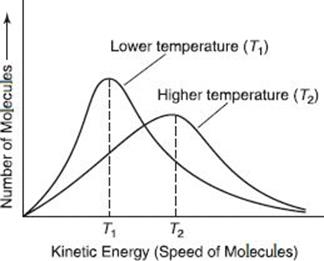
figure: Molecular Speed Distribution in a Gas at Different Temperatures
When the temperature is lowered, the gas reaches a point at which the kinetic energy can no longer overcome the attractive forces between the particles (or molecules) and the gas condenses to a liquid.The temperature at which this condensation occurs is related to the type of substance the gas is composed of and the type of bonding in the molecules themselves.
the figure above主要是想要講甚麼 在low temperature, kinetic energy 會比較大curve的變化較大為甚麼不是在high temperature?
T1比較高位甚麼是LOW temperature T2比較低位甚麼是high temperature 怎麼說明kinetic energy,temperature and number of moelcules的change and relationships
the paragraph below 可以先提共翻譯在解釋嗎(關於上圖的敘述)
謝謝
